Zinc Carbonate And Hydrochloric Acid Equation . Znco3 → zno + co2. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. The chemical reaction is as below. Acid + carbonate salt + co 2 + water. Znco3 + 2hcl → zncl2 + co2 + h2o. — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Acid + metal → salt + hydrogen hydrochloric. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide.
from www.numerade.com
Znco3 → zno + co2. Znco3 + 2hcl → zncl2 + co2 + h2o. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. Acid + metal → salt + hydrogen hydrochloric. Acid + carbonate salt + co 2 + water. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide.
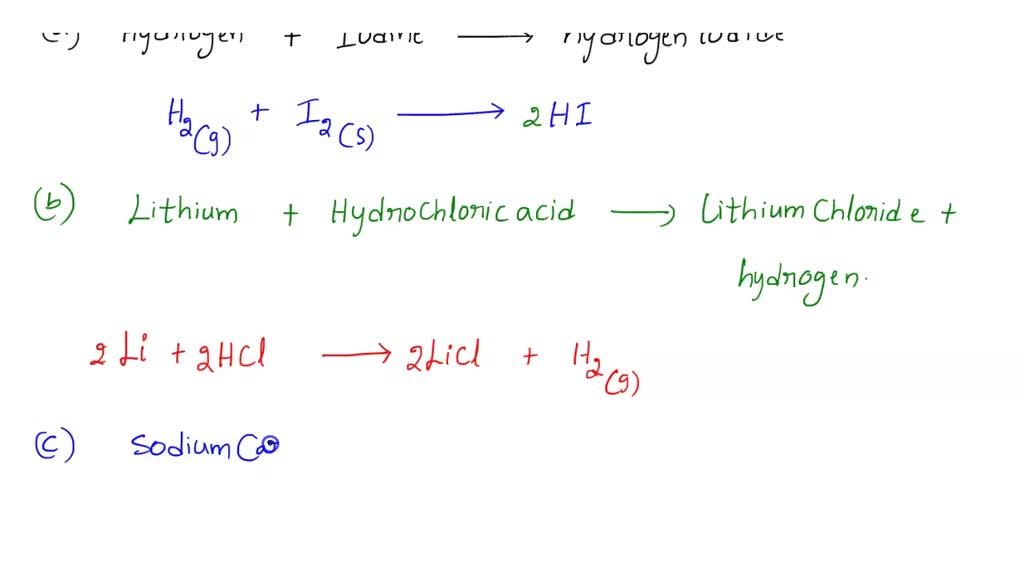
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER SODIUM SOLID IODINE SOLID
Zinc Carbonate And Hydrochloric Acid Equation name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 + 2hcl → zncl2 + co2 + h2o. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Acid + metal → salt + hydrogen hydrochloric. The chemical reaction is as below. Znco3 → zno + co2. Acid + carbonate salt + co 2 + water. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the of Zinc Zinc Carbonate And Hydrochloric Acid Equation Acid + carbonate salt + co 2 + water. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. — zinc carbonate reacts with acids like hydrochloric acid to form zinc. Zinc Carbonate And Hydrochloric Acid Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc Carbonate And Hydrochloric Acid Equation Znco3 + 2hcl → zncl2 + co2 + h2o. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. . Zinc Carbonate And Hydrochloric Acid Equation.
From www.toppr.com
Write balanced chemical equations for the followinga) Calcium carbonate reacts with Zinc Carbonate And Hydrochloric Acid Equation — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for. Zinc Carbonate And Hydrochloric Acid Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Carbonate And Hydrochloric Acid Equation Znco3 → zno + co2. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Acid + metal → salt + hydrogen hydrochloric. — in this video we'll balance. Zinc Carbonate And Hydrochloric Acid Equation.
From dxodpvcix.blob.core.windows.net
Zinc Carbonate Ionic Or Molecular at Corrina Yan blog Zinc Carbonate And Hydrochloric Acid Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. The chemical reaction is as below. Acid + metal → salt + hydrogen hydrochloric. Zinc carbonate undergoes decomposition forming zinc. Zinc Carbonate And Hydrochloric Acid Equation.
From www.numerade.com
SOLVEDWrite net ionic equations for the following reactions (a) The reaction of acetic acid, a Zinc Carbonate And Hydrochloric Acid Equation — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Acid + carbonate salt + co 2 + water. Znco3 → zno + co2. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. — zinc carbonate reacts with acids like hydrochloric acid to form. Zinc Carbonate And Hydrochloric Acid Equation.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq.) ZnCl2(aq.)+ H2(9) If 0. Zinc Carbonate And Hydrochloric Acid Equation — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. The chemical reaction is as below. Znco3 + 2hcl → zncl2 + co2 + h2o. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. — zinc carbonate reacts with acids like hydrochloric. Zinc Carbonate And Hydrochloric Acid Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Carbonate And Hydrochloric Acid Equation Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Znco3 + 2hcl → zncl2 + co2 + h2o. Acid + metal → salt + hydrogen hydrochloric. The chemical reaction is as below. Znco3 → zno + co2. — in this. Zinc Carbonate And Hydrochloric Acid Equation.
From yhss-3e5-chemistry-21-2011.blogspot.com
chemistry Zinc Carbonate And Hydrochloric Acid Equation Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Acid + metal → salt + hydrogen hydrochloric. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. Acid + carbonate salt + co 2 + water. name the products of the reaction between zinc carbonate and. Zinc Carbonate And Hydrochloric Acid Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Of Power Zinc Carbonate And Hydrochloric Acid Equation Znco3 → zno + co2. The chemical reaction is as below. Acid + metal → salt + hydrogen hydrochloric. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. . Zinc Carbonate And Hydrochloric Acid Equation.
From dxorwmpbw.blob.core.windows.net
Word Equation Between Zinc And Hydrochloric Acid at Noah Brooks blog Zinc Carbonate And Hydrochloric Acid Equation Acid + carbonate salt + co 2 + water. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Znco3 → zno + co2. Zinc carbonate undergoes decomposition forming zinc. Zinc Carbonate And Hydrochloric Acid Equation.
From www.youtube.com
How to Write the Formula for Zinc bicarbonate (Zinc hydrogen carbonate) YouTube Zinc Carbonate And Hydrochloric Acid Equation — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Acid + metal → salt + hydrogen hydrochloric. Acid + carbonate salt + co 2 + water. Znco3 → zno + co2. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in. Zinc Carbonate And Hydrochloric Acid Equation.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Zinc Carbonate And Hydrochloric Acid Equation — zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. Znco3 → zno + co2. The chemical reaction is as below. Acid + metal → salt + hydrogen hydrochloric. . Zinc Carbonate And Hydrochloric Acid Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Carbonate And Hydrochloric Acid Equation name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + carbonate salt + co 2 + water. for reactions of these acids with iron or zinc, the. Zinc Carbonate And Hydrochloric Acid Equation.
From dxorjadau.blob.core.windows.net
Zinc Carbonate Equation at Mary Wallace blog Zinc Carbonate And Hydrochloric Acid Equation — in this video we'll balance the equation hcl + znco3 = zncl2 + co2 + h2o. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Znco3 + 2hcl → zncl2 + co2 + h2o. name the products of the reaction between zinc carbonate and sulfuric acid, and write. Zinc Carbonate And Hydrochloric Acid Equation.
From dxojlqivq.blob.core.windows.net
Zinc Carbonate Individual Ions at Tina Sanchez blog Zinc Carbonate And Hydrochloric Acid Equation Znco3 → zno + co2. The chemical reaction is as below. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. — zinc carbonate reacts with acids like hydrochloric. Zinc Carbonate And Hydrochloric Acid Equation.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when excess hydrochloric acid Zinc Carbonate And Hydrochloric Acid Equation name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.. Zinc Carbonate And Hydrochloric Acid Equation.
From www.tessshebaylo.com
Chemical Equation For Sodium Hydrogen Carbonate And Hydrochloric Acid Tessshebaylo Zinc Carbonate And Hydrochloric Acid Equation The chemical reaction is as below. — for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 + 2hcl → zncl2 + co2 + h2o. for reactions of these acids with iron or zinc, the students simply substitute fe or zn for mg in these equations. Zinc carbonate undergoes decomposition forming zinc oxide. Zinc Carbonate And Hydrochloric Acid Equation.